United States Centers for Medicare & Medicaid Services (CMS) - Division of Clinical Laboratory Improvement & Quality
Please Note
We are a service company that can help you file with the United States Centers for Medicare & Medicaid Services (CMS) - Division of Clinical Laboratory Improvement & Quality. We are not associated with this nor any other government agency. We offer paid services and software to help you file. You are not required to purchase our service to file - you may file directly with this agency without using our service.
Contact Information
Licenses
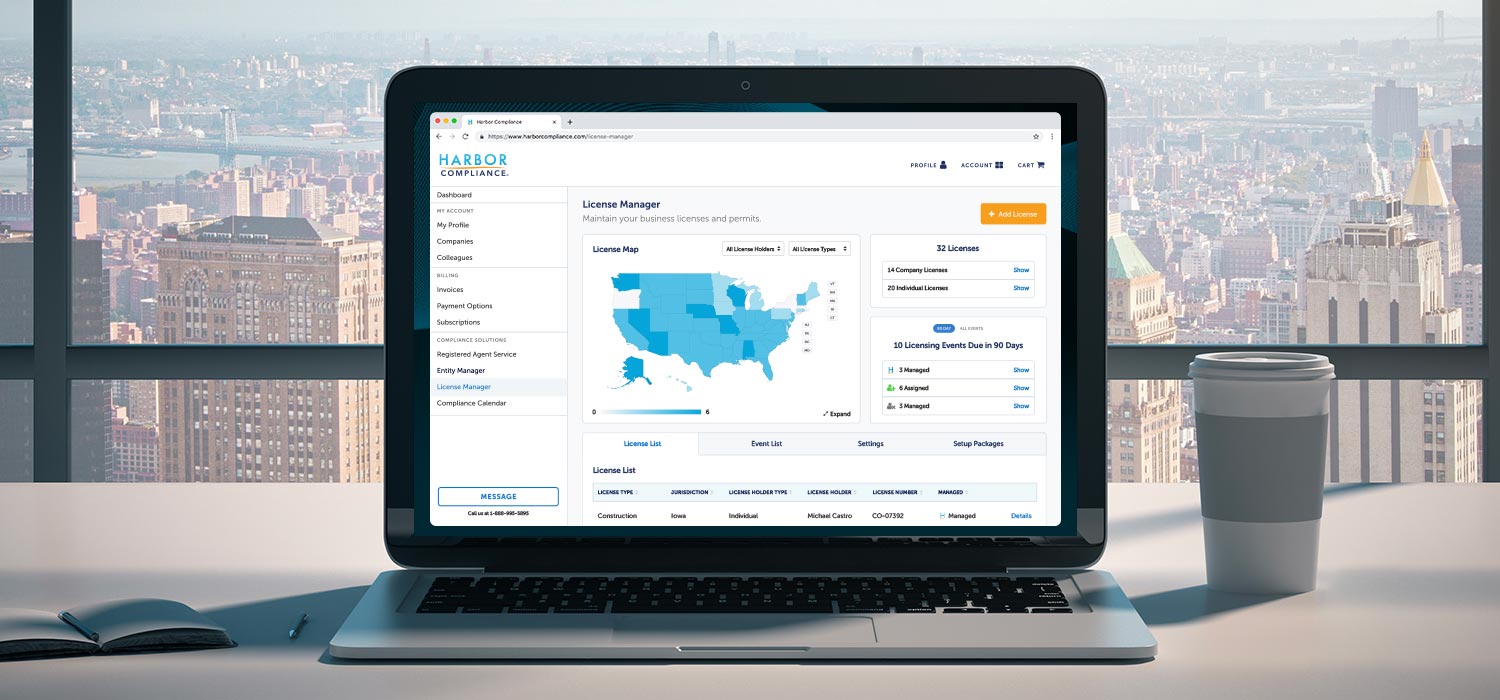
We track the following licenses with the United States Centers for Medicare & Medicaid Services (CMS) - Division of Clinical Laboratory Improvement & Quality in order to provide compliance services to our clients. As a client, you see this and other Compliance Core™ data in License Manager in-line with your licenses.
United States Clinical Laboratory Improvement Amendments (CLIA) Certificate for Provider Performed Microscopy Procedures
Initial Registration
| Form: | Form CMS 116: Clinical Laboratory Improvement Amendments (CLIA) Application for Certification |
| Agency Fee: | $240, invoiced after approval |
| Notes: | Applications should be submitted to the state agency which handles CLIA Certificate applications for the state in which your laboratory resides. See the list of CLIA State Agency Contacts for contact information. |
Registration Renewal
| Agency Fee: | $240 |
| Due: | Biennially |
| Notes: | There is no renewal application, simply submit the fee after receiving the invoice. |
United States Clinical Laboratory Improvement Amendments (CLIA) Certificate of Accreditation
Initial Registration
| Form: | Form CMS 116: Clinical Laboratory Improvement Amendments (CLIA) Application for Certification |
| Agency Fee: | Varies by type of laboratory and annual test volume. |
| Notes: | Applications should be submitted to the state agency which handles CLIA Certificate applications for the state in which your laboratory resides. See the list of CLIA State Agency Contacts for contact information. |
Registration Renewal
| Agency Fee: | Varies by type of laboratory and annual test volume. |
| Due: | Biennially |
| Notes: | There is no renewal application, simply submit the fee after receiving the invoice. |
United States Clinical Laboratory Improvement Amendments (CLIA) Certificate of Compliance
Initial Registration
| Form: | Form CMS 116: Clinical Laboratory Improvement Amendments (CLIA) Application for Certification |
| Agency Fee: | Varies by type of laboratory and annual test volume. |
| Notes: | Applications should be submitted to the state agency which handles CLIA Certificate applications for the state in which your laboratory resides. See the list of CLIA State Agency Contacts for contact information. |
Registration Renewal
| Agency Fee: | Varies by type of laboratory and annual test volume. |
| Due: | Biennially |
| Notes: | There is no renewal application, simply submit the fee after receiving the invoice. |
United States Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver
Initial Registration
| Form: | Form CMS 116: Clinical Laboratory Improvement Amendments (CLIA) Application for Certification |
| Agency Fee: | $180, invoiced after approval |
| Notes: | Applications should be submitted to the state agency which handles CLIA Certificate applications for the state in which your laboratory resides. See the list of CLIA State Agency Contacts for contact information. |
Registration Renewal
| Agency Fee: | $180 |
| Due: | Biennially |
| Notes: | There is no renewal application, simply submit the fee after receiving the invoice. |
Related Government Agencies
Explore other United States government agencies we can help you stay compliant with:
- Administrative Office of the U.S. Court
- Federal Motor Carrier Safety Administration (FMCSA)
- Internal Revenue Service (IRS)
- National Oceanic and Atmospheric Administration (NOAA) - Office of Sustainable Fisheries
- U.S Department of State - Bureau of Consular Affairs - Office of Authentications
- U.S. Department of Homeland Security (DHS)
- U.S. Department of Housing and Urban Development
- U.S. Department of Justice - Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF)
- U.S. Department of Justice (DOJ)
- U.S. Department of Labor (DOL)
- U.S. Department of the Treasury
- U.S. Department of the Treasury - Alcohol and Tobacco Tax and Trade Bureau (TTB)
- U.S. Department of the Treasury - Financial Crimes Enforcement Network (FinCEN)
- U.S. Department of Transportation (DOT)
- U.S. Department of Transportation (DOT) - Pipeline and Hazardous Materials Safety Administration (PHMSA)
- U.S. Drug Enforcement Administration (DEA) - Diversion Control Division
- U.S. Environmental Protection Agency
- U.S. Environmental Protection Agency - Lead Paint Program
- U.S. Federal Communications Commission (FCC)
- U.S. Federal Deposit Insurance Corporation (FDIC)
- U.S. Federal Trade Commission (FTC)
- U.S. Fish and Wildlife Service (FWS)
- U.S. National Credit Union Administration (NCUA)
- U.S. Occupational Safety and Health Administration (OSHA)
- U.S. Patent and Trademark Office (USPTO) - Trademarks
- U.S. Securities and Exchange Commission (SEC)
- U.S. Small Business Administration (SBA)
- United States Centers for Medicare & Medicaid Services (CMS)
- USDA - Animal and Plant Health Inspection Service (APHIS)





